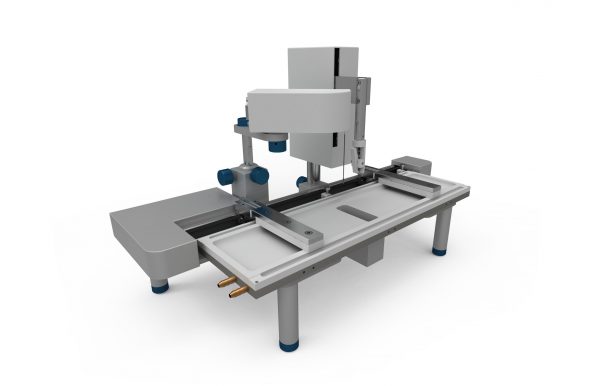
KSV NIMA Langmuir and Langmuir-Blodgett Troughs (KSV NIMA L & LB Troughs) are used to fabricate and characterize monomolecular films with precise control of lateral packing density.
Following characterization studies of the unique properties of molecules in monolayers, the instruments can also be used to transfer these monolayers using a Langmuir-Blodgett or Langmuir-Schaefer deposition technique. This enables the creation of single and multi-layer films with precise control of thickness, molecular orientation and packing density.
Features & Benefits
- Full control over your experiment:
- Powerful and intuitive software satisfying novice and experienced user’s needs. See detailsbelow.
- Ultra-sensitive surface pressure sensor for extremely precise measurements. Platinum plates, platinum rod and paper plates can be used as probe to meet all needs.
- Open design and modularity enables easy placement of trough tops into the frame allowing substitution with another trough top within seconds and easy cleaning of the trough top surface.
- Langmuir and Langmuir Blodgett trough tops are made from single pieces of pure PTFE for optimized cleanliness and reliability. This unique design prevents any leakage in any part of the trough top including the dipping well. It avoids the use of potentially contaminating glues or other seals.
- Compression barriers are made from Delrin (hydrophilic), for enhanced monolayer stability. PTFE (hydrophobic) compression barriers can also be supplied upon request. A robust metal frame prevents barriers from deforming over time.
- Thin frame design allowing combination of optical characterization techniques such as PM-IRRAS(infrared spectroscopy), Brewster Angle Microscopy, and fluorescence microscopy etc.
- Symmetric barrier compression as a standard for homogenous packing. Single barrier compression is also available with every instrument.
- Centered dipping well allowing uniform monolayer LB deposition.
- Sub-phase temperature control facilitated by aluminum heat/cool base plate operated by external circulating water bath (the water bath is sold separately).
- Adjustable legs enable fast and accurate leveling of the trough. The legs can be removed easily for to place the trough on a microscope.
- Wide range of accessories (Horizontal dipping clamps, Surface Potential Meter, pH probe, etc.)
- KSV NIMA’s extensive knowledge and application support team let you get the best out of your instrument.
- Highly modular thanks to a unique exchangeable trough top mechanism that allows combining all Extra Small, Small and Medium trough tops from KSV NIMA Langmuir and Langmuir-Blodgett selection. You can change functionality of your system, at any time: for instance, exchanging a Langmuir trough top to a LB trough top, or selecting a different size of trough top. All systems except the Alternate are compatible (directly or after upgrade with a new trough top) with KSV NIMA Surface Potential Sensor,Brewster Angle Microscopes (KSV NIMA BAM and/or KSV NIMA MicroBAM) and PM-IRRAScharacterization instruments.
The most popular L & LB trough systems are presented in the following table. Other combinations are possible. To find out more—contact your nearest KSV NIMA representative.
KSV NIMA Langmuir Troughs
Standard Langmuir Troughs are available in several sizes: Extra Small (lower volume but larger area than Small), Small, Medium and Large. It is important to stress that all systems can be easily switched between the Langmuir, Langmuir-Blodgett and Microscopy configurations. The three smaller troughs use the same frame providing the flexibility to change the trough top size at any time.
High Compression Trough enables high compression ratios and is specifically designed for the KSV NIMAInterfacial Shear Rheometer (ISR). The High Compression Trough can also be used for increased performance with Brewster Angle Microscope (MicroBAM), Surface Potential Sensor, PM-IRRAS and other characterization instruments.
The Liquid-Liquid Langmuir Trough is available for monolayer studies at the liquid-liquid interface (typically, oil-water). The Liquid-Liquid Trough top is also compatible with the High Compression Trough.
The KSV NIMA Microscopy Trough is a special kind of Langmuir Trough, which contains a sapphire window in the trough top base allowing high optical transmission down to a wavelength of 200 nm (suitable for visible light or UV microscopy). The Medium and the High Compression Troughs are both suitable for upright and inverted microscopy. The Small Trough can be used for upright microscopy.
For more information about Langmuir film microscopy, see:
Langmuir Film Microscopy
The Langmuir Ribbon Barrier Trough enables the study of floating monolayers at high packing densities (e.g. > 70 mN/m for DPPC) by monolayer confinement. Working at high surface pressures is required to study phenomena such as lung surfactant (DPPC) behavior in alveoli.
KSV NIMA Langmuir-Blodgett Troughs
The KSV NIMA LB Trough is also available in several sizes (Small, Medium and Large) and includes the same system flexibility between the Langmuir, Microscopy and Ribbon Barrier configurations.
You can deposit LB films on samples ranging in size from a few square millimeters to many tens of square centimeters. Dipping well dimensions, and hence suitable substrate areas, are dependent on the model of trough and trough tops (see Specification Table). The LB dipping mechanism can also be fitted with a LS deposition kit for horizontal Langmuir-Schaefer Deposition.
The KSV NIMA Alternate Layer Deposition Trough enables simultaneous creation of two Langmuir films in two separate compartments. The sample can be moved through any of the two monolayers in the desired order. It is available in two sizes—standard and large.
Many experimental techniques can be used to further investigate monolayers at the gas-liquid interface even before deposition, including Interfacial Shear Rheometry, PM-IRRAS, Brewster Angle Microscopy andSurface Potential Sensing.
KSV NIMA LB Software
The KSV NIMA LB software is very intuitive and easy to use. It allows the user to perform a variety of pre-programmed methods that cover the most common L and LB film experiments. These pre-programs can be modified further for particular needs. A wide range of data and parameters can be recorded and the desired data can be easily plotted. The recordable parameters are: data point number, time, barrier position, barrier speed, trough top area, molecular area, dipper position, dipper speed, layer number, transfer ratio, cumulative transfer, temperature, pH and surface potential.
Standard programs include:
|
|
Compression/relaxation isotherms
|
Measuring surface pressure as a function of mean molecular area, remaining area, time or any other measured parameter. |
Analysis of monolayer kinetics
|
Enzyme kinetics, monolayer hydrolysis, polymerization, or any other zero-order reactions. |
Analysis of monolayer penetration, solubility and binding of biomolecules
|
Enzymes, proteins, peptides etc. |
Isochores and Isobars
|
Increase or decrease of surface pressure/temperature, surface pressure/time, or surface pressure/any desired measurable parameter can be plotted. |
Dilational rheology
|
Oscillating barriers for monitoring viscoelastic properties at desired surface pressure. |
Dipping
|
Both Langmuir-Blodgett and Langmuir-Schaefer modes allow the control and monitoring of surface pressure, dipping speed, stroke length, deposition profiles and transfer ratio. |
After an experiment has been performed the user can return to the data for further analysis in the data reduction and analysis section. After selecting an experiment the data for that experiment will be displayed. Different experimental data can be displayed on the same graph for comparison. Calculation of additional results and export of data can be done. There is an option of viewing and editing the experimental setup if the data needs to be recalculated with new information about the materials.
Mọi chi tiết vui lòng liên hệ với chúng thôi theo thông tin:
CÔNG TY TNHH VINTEK
Địa chỉ: 280/130 Bùi Hữu Nghĩa, Phường 2, Quận Bình Thạnh, Thành phố Hồ Chí Minh
Điện thoại: 0913146368
Email: vintek-info@vintekco.com
Langmuir Troughs & Langmuir-Blodgett Troughs
Biomembranes and biomolecular interactions such as cell membrane models, conformational changes and reactions, drug delivery.
Functional and novel coatings of nanoparticles, nanotubes, nanowires, graphene.
Interfacial reactions such as polymerization, immunological and enzymatic reactions, biosensors, adsorption and desorption.
Surfactants and colloids research including molecule structure and orientation characterization, formulation, emulsion, colloid and foam stability.
Rheology of thin films such as, dilational rheology, interfacial shear rheology with the KSV NIMA ISR.
Application Examples
Membrane models: Case study II: Chitosan as a removing agent of β-Lactoglobulin from membrane models
Lipophilic proteins often reside in cell membranes, and the floating monolayer models can be used for studying their interactions in a close to native environment. The removal of allergenic proteins from food is a highly beneficial technology for food processing in order to produce healthy foods like milk products for people who are allergic to some components in them. Aside from lactose intolerance, allergy to β-Lactoglobulin found in milk can prevent consumption of milk products. In the following example chitosan was studied as a removal agent for β-Lactoglobulin using the KSV NIMA PM-IRRAS and a KSV NIMA Langmuir Trough.
Caseli et al., ‘Langmuir’ 24 (2008), 4150-4156
Build-up of layer-by-layer films: monitoring of Metal-Organic framework assembly
Dip coating, LB and LS techniques have shown to be excellent methods for controlled preparation of thin films of graphene and graphene oxide. These methods offer a great amount of control for depositing dispersed SG and SGO produced by the liquid exfoliation methods. As the liquid exfoliation methods are recognized as some of the most potential methods for producing graphene at an industrial scale, these deposition methods are of great importance in graphene research.
With PM-IRRAS it was possible to record detailed IR spectra of floating and deposited layers to determine the chemical composition. It was possible to create a single sheet of graphene, validating LS as a good method to create and study single sheet graphene and graphene oxide films.


 (028) 22 611 711
(028) 22 611 711
 Danh mục
Danh mục
 High Purity Northwest Inc.
High Purity Northwest Inc.
 Phóng Điện Cục Bộ
Phóng Điện Cục Bộ































 Gọi điện
Gọi điện SMS
SMS Chỉ Đường
Chỉ Đường